Reactions of osmapyridinium with terminal alkynes†
Abstract
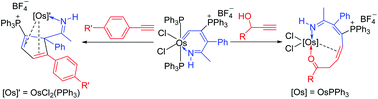
We have synthesized a new type of ten-membered osmacycles by reaction of osmapyridinium with HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CCH(OH)R (R = Ph, Et). We propose that these reactions take place initially by coordination of the alkynes, [2 + 2] cycloaddition, subsequent 1,2-hydrogen migration and a final reductive elimination. The reactions with phenylacetylenes do not afford the corresponding derivatives but rather give η4-coordinated cyclopentadiene complexes, which are proposed to derive from a [4 + 2] cycloaddition process. Related reactions of the η4-coordinated cyclopentadiene complexes are also discussed.
CCH(OH)R (R = Ph, Et). We propose that these reactions take place initially by coordination of the alkynes, [2 + 2] cycloaddition, subsequent 1,2-hydrogen migration and a final reductive elimination. The reactions with phenylacetylenes do not afford the corresponding derivatives but rather give η4-coordinated cyclopentadiene complexes, which are proposed to derive from a [4 + 2] cycloaddition process. Related reactions of the η4-coordinated cyclopentadiene complexes are also discussed.

 Please wait while we load your content...
Please wait while we load your content...