Zinc diiodide-promoted synthesis of trisubstituted allenes from propargylic amines†
Abstract
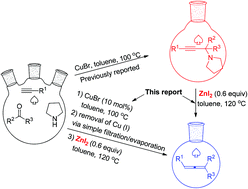
Zinc diiodide has been identified as an effective reagent for the efficient synthesis of trisubstituted allenes from propargylic amines. Compared to CdI2 this protocol offers a green approach. Due to the easy preparation of propargylic amines through the method developed by this group, this method provides a two-step synthesis of trisubstituted allenes from 1-alkynes, ketones, and pyrrolidine. Finally, an efficient synthesis of such trisubstituted allenes from 1-alkynes, ketones, and pyrrolidine via simple filtration has been developed. Compared with the CdI2-mediated protocol, the current protocol enjoys a much wider scope for ketones and affords functionalized allenes without further cyclization in some substrates.

 Please wait while we load your content...
Please wait while we load your content...