An efficient synthesis of gem-diiodoolefins and (E)-iodoalkenes from propargylic amides with a Cu(i)/Cu(iii) cycle†
Abstract
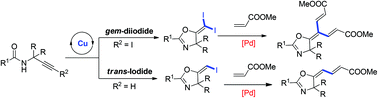
gem-Dihaloalkenes are very important building blocks widely used in organic synthesis. However, the synthetic methods available for this functional group are very limited. Starting from an unexpected copper-catalyzed cyclization of propargylic amides, a new route for the synthesis of gem-dihaloalkenes was developed. According to the plausible Cu(I)/Cu(III) catalytic cycle, another oxidative iodination protocol was developed. Through this improved method, a series of gem-diiodoolefins were synthesized in high yields. In addition, (E)-iodoalkenes could also be synthesized efficiently using the same approach, which is complementary to the gold-catalyzed reaction giving (Z)-iodoalkenes. These iodoolefins could be further transformed into various trisubstituted or tetrasubstituted alkenes, enynes, dienes and trienes with palladium-catalyzed coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...