Alkyl- and aryl-thioalkylation of olefins with organotrifluoroborates by photoredox catalysis†
Abstract
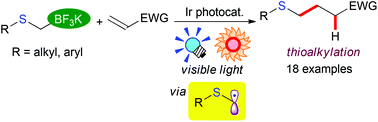
A facile and environmentally benign protocol for alkyl- and aryl-thioalkylation of olefins has been developed. Photoredox catalysis with an Ir photocatalyst, [Ir(dF(CF3)ppy)2(bpy)](PF6) (dF(CF3)ppy: 5-trifluoromethyl-2-(2,4-difluorophenyl)pyridine, bpy: 2,2′-bipyridine), induces efficient oxidation of a variety of alkyl- and aryl-thioalkyltrifluoroborates under visible light irradiation at room temperature, leading to the generation of α-thioalkyl radicals via deboronation. The generated α-thioalkyl radicals smoothly react with electron-deficient olefins to afford addition products in good yields. The present photocatalytic method provides us with simple and new access to a range of alkylsulphides under mild reaction conditions.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2015

 Please wait while we load your content...
Please wait while we load your content...