NIS/CHP-mediated reaction of isocyanides with hydrazones: access to aminopyrazoles†
Abstract
A NIS/CHP-mediated [4 + 1]-cycloaddition of isocyanides with 1,2-diaza-1,3-dienes generated in situ from hydrazones under metal-free conditions has been developed. This protocol provides a new, atom efficient and step efficient way to construct aminopyrazoles in good to excellent yields via formation of new C–C/C–N bonds, utilizing a catalytic amount of NIS in the presence of CHP.
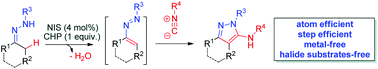

 Please wait while we load your content...
Please wait while we load your content...