Gold-catalyzed highly efficient benzylation of alcohols with N-Cbz-N-benzyl-propargylamine†
Abstract
N-Cbz-N-benzyl-propargylamine is easily synthesized and described as a benzylating reagent. The treatment of the novel reagent with various alcohols in the presence of the IPrAuNTf2 catalyst affords benzyl ethers in high yields. The protocol derives practical benefits from the mild conditions and the high functional group tolerance, and also eliminates the need for excess base additives or moisture-sensitive promoters.
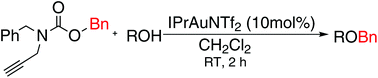

 Please wait while we load your content...
Please wait while we load your content...