1,2-Alkylarylation of activated alkenes with dual C–H bonds of arenes and alkyl halides toward polyhalo-substituted oxindoles†
Abstract
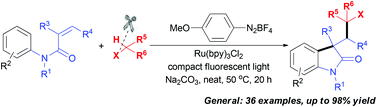
We describe here a new visible light facilitated radical strategy for 1,2-alkylarylation of activated alkenes with a C(sp2)–H bond of arenes and a C(sp3)–H bond of alkyl halides. This method achieves selective scission of the C(sp3)–H bond adjacent to halide atoms leading to a halo-substituted alkyl radical, and provides a new synthetic utilization of aryl halides toward polyhalo-substituted oxindoles in good to excellent yields. Moreover, the concise transformation of the products, polyhalo-substituted oxindoles, into vinyl halides and alkynyl halides was also illustrated.

 Please wait while we load your content...
Please wait while we load your content...