Morphology–activity correlation in hydrogen evolution catalyzed by cobalt sulfides†
Abstract
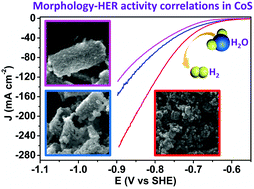
Transition metal chalcogenides such as cobalt sulfides (CoS) have recently attracted significant interest in electrocatalytic hydrogen evolution reaction (HER). In addition to the constituent elements and hence intrinsic activity, the morphology, porosity, and specific surface area of a nanostructured catalyst would substantially impact its overall electrocatalytic performance. In this paper, we report a facile and rapid two-step microwave-assisted anion-exchange route to prepare nanostructured CoS. By simply controlling the microwave sulfurization time, CoS of various morphologies such as hollow prisms, broken prisms, and nanoparticles could be obtained. Importantly, the correlation between morphology and HER activity of CoS in neutral water was systematically studied through a set of material characterization and electrochemical techniques. It's revealed that the morphology of CoS changed from hollow nanoprisms to 3D nanoparticles when increasing the microwave sulfurization time from 5 to 60 min. The results demonstrated that CoS with 3D nanoparticle morphology, prepared by microwave sulfurization of 30 min, possessed the largest specific surface area and electrochemically active surface area. These nanostructured features resulted in the promoted accessibility of active sites, enhanced mass/charge transport and easier release of hydrogen bubbles, rendering its highest HER activity and excellent stability and showing small overpotentials of 233, 314, and 364 mV to achieve current densities of 10, 50, and 100 mA cm−2, respectively, in neutral water.
- This article is part of the themed collection: 2015 Emerging Investigators by ICF

 Please wait while we load your content...
Please wait while we load your content...