Inorganic anion-assisted supramolecular assemblies of bent dipyridines: effects of anionic geometries on hydrogen-bonding networks†
Abstract
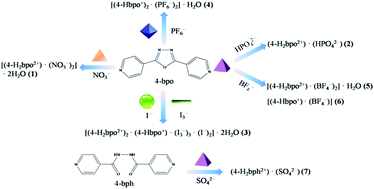
A series of inorganic acids were introduced into the self-assembly with the bent 2,5-bis(4-pyridyl)-1,3,4-oxadiazole (4-bpo), yielding seven anion-assisted supramolecular salts, i.e. [(4-H2bpo2+)·(NO3−)2]·2H2O (1), (4-H2bpo2+)·(HPO42−) (2), [(4-H2bpo2+)2·(4-Hbpo+)·(I3−)3·(I−)2]·2H2O (3), [(4-Hbpo+)2·(PF6−)2]·H2O (4), [(4-H2bpo2+)·(BF4−)2]·H2O (5), [(4-Hbpo+)·(BF4−)] (6) and (4-H2bph2+)·(SO42−) (7). Structural analyses indicate that different inorganic anions (e.g. spherical, linear, trigonal planar, tetrahedral and octahedral) can induce the formation of diverse supramolecular architectures, influencing the crystallization ratio, the protonated number and the final structure. The anions are hydrogen bonded to the angular dipyridine, which offers a sufficient driving force for the directed assembly of supramolecular hydrogen-bonding frameworks. Thereto, various hydrogen-bonding motifs are observed in all these cases (1–7) and the nitrogen atoms of pyridines are protonated apart from salt 6. Among them, salts 1, 3, 4 and 5 crystallize with water molecules but the others do not. Interestingly, appealing substructures have been generated by anions and water molecules in 1, 3 and 5 but not in 4. HPO42− dimers form in 2 despite there being no assistance from solvent water. BF4− anions facilitate the formation of the helical chain in 6. Unexpectedly, the oxadiazole ring opened via the in situ hydrolysis under hydrothermal conditions during crystallization with H2SO4, producing salt 7.

 Please wait while we load your content...
Please wait while we load your content...