Dibenzoyldiethylgermane as a visible light photo-reducing agent for CuAAC click reactions
Abstract
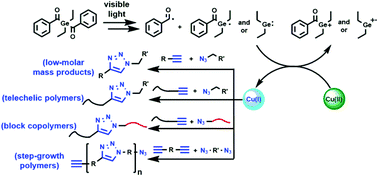
A highly active, versatile and photoresponsive system for copper catalyzed azide–alkyne cycloaddition (CuAAC) click reaction has been developed using the dibenzoyldiethylgermane (DBDEG) photoinitiator with the copper(II) chloride (CuCl2)–N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA) ligand under visible light. Selected azide and alkyne compounds with various functional groups are used to conduct the click reaction and almost quantitative yields are attained. The approach pertains to the visible light generation of germyl radicals capable of reducing Cu(II) ions to Cu(I) species to catalyze the CuAAC reactions. This strategy has been applied in the achievement of various macromolecular reactions including polymer end-group functionalization, block copolymer formation and step-growth polymerization.

 Please wait while we load your content...
Please wait while we load your content...