Regio- and stereoselective construction of stimuli-responsive macromolecules by a sequential coupling-hydroamination polymerization route†
Abstract
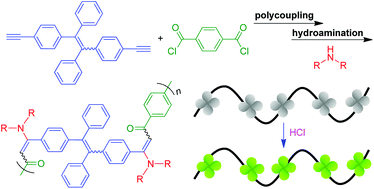
Herein we reported a new facile one-pot multicomponent sequential polymerization approach for the construction of conjugated nitrogen-substituted polymers. Catalyzed by Pd(PPh3)2Cl2/CuI at room temperature, the coupling-hydroamination polymerizations of 1,2-bis(4-ethynylphenyl)-1,2-diphenylethene, terephthaloyl chloride and secondary aliphatic amines proceeded smoothly in a regioregular and stereoselective manner, furnishing poly(arylene enaminone)s (PAEs) with high molecular weights (Mw up to 34 600) in satisfactory yields (up to 91%). A model compound was elaborately designed and synthesized to verify the chemical structures of the corresponding polymeric products. All the PAEs exhibited good solubility in common organic solvents and were thermally stable with degradation temperatures of up to 313 °C under nitrogen. They possessed good film-forming ability and their thin solid films showed high refractive indices (RI = 1.9318–1.6320) in a wide wavelength region of 400–1000 nm, whose value could be further modulated by UV irradiation. Although the model compound and the PAEs possessed a typical aggregation-induced emission luminogen of tetraphenylethene, they were weakly emissive either in solution or in the aggregated state, due to the photoinduced electron transfer (PET) effect. Their strong emission in the aggregated state could be readily recovered by the blockage of the PET effect through protonation of the amino groups. Thus, this work demonstrated a powerful polymerization tool to access conjugated polymeric materials with pH-responsive properties.

 Please wait while we load your content...
Please wait while we load your content...