Energetic polymeric networks prepared via a solvent- and catalyst-free thermal cycloaddition of azide-bearing polymers with alkynes and hydroxyl-isocyanate addition reactions†
Abstract
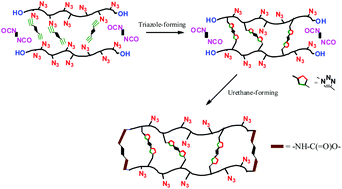
Polymeric networks were prepared through a 1,3-dipolar cycloaddition of azide-bearing polymers with a variety of compounds having two or three terminal alkynes without solvents and catalysts. Dipolarophiles with an α-carbonyl underwent a very rapid Huisgen reaction within a few minutes to afford networks that were side-linked with triazole moieties. The reactivities of dipolarophiles were estimated by using frontier molecular orbital energies. To avoid the formation of defects in elastically ineffective networks, all polymer chain-ends were linked with urethane moieties, and very small quantities of azides were reacted with the dipolarophiles to link the pendent groups with the triazoles. Because the crosslinking densities of the energetic networks were inversely proportional to the reactivity of the dipolarophiles to the azides, the less reactive alkynes provided better mechanical properties to the networks prepared using the Huisgen cycloaddition reaction compared to the more reactive alkynes.

 Please wait while we load your content...
Please wait while we load your content...