Alternating copolymers of functionalized α-methyl styrene monomers and maleic anhydride†
Abstract
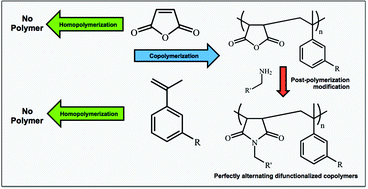
Alkyl and tertiary amine functionalized α-methyl styrene (AMS) monomers have been synthesized via reactive coupling of 3-isopropenyl-α,α-dimethylbenzyl isocyanate (TMI) with primary amines. Primary amines utilized include hexylamine, octadecylamine and N,N-dimethylethylenediamine to synthesize monomers, 3-hexyl-1-[1-(m-isopropenylphenyl)-1-methylethyl]urea (AMSC6), 3-octadecyl-1-[1-(m-isopropenylphenyl)-1-methylethyl]urea (AMSC18) and 3-(2-(dimethylamino)ethyl)-1-[1-(m-isopropenylphenyl)-1-methylethyl]urea (AMSDMA), respectively. The structures of these functionalized AMS monomers have been confirmed by 1H-NMR, 13C-NMR and FT-IR. AMS, AMSC6 or AMSC18 was then successfully copolymerized with maleic anhydride (MalA) via free radical polymerization initiated by azobisisobutyronitrile to synthesize alternating copolymers. Free radical homopolymerizations of AMS, AMSC6, AMSC18 and MalA were performed to reveal no monomer conversion by gas chromatography and no formation of polymer chains by gel permeation chromatography (GPC). Alternating copolymers were characterized by GPC, 1H-NMR, FT-IR and MALDI-TOF MS. Finally, post-polymerization modification of P[(AMSC18)-a-(MalA)] by imidization of the MalA repeat unit with a primary amine was performed. This leads to the synthesis of alternating dual functionalized copolymers in an efficient and simple way.

 Please wait while we load your content...
Please wait while we load your content...