Polyhydroxyalkanoate-based amphiphilic diblock copolymers as original biocompatible nanovectors†
Abstract
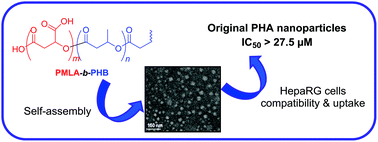
Polyhydroxyalkanoate-based diblock copolymers, poly(β-malic acid)-b-poly(3-hydroxybutyrate) (PMLA-b-PHB), featuring different sizes of segments have been synthesized, for the first time, in two steps and in grams quantity. First, the controlled sequential ring-opening polymerization of β-butyrolactone (BL, in bulk) followed by benzyl β-malolactonate (MLABe, in toluene) has been achieved with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) to afford well-defined poly(benzyl β-malolactonate)-b-poly(3-hydroxybutyrate) (PMLABe-b-PHB) copolymers. A subsequent hydrogenolysis of β-benzyloxycarbonyl units afforded the corresponding PMLA-b-PHB amphiphilic copolymers, as evidenced by 1H, 13C{1H} and DOSY NMR, SEC, TGA, DSC and contact angle analyses. Next, PMLA-b-PHB copolymers featuring different hydrophilic weight fractions (10–65%) self-assembled in PBS upon nanoprecipitation. Nano-objects with narrow size distributions (Dh = 17–180 nm; 0.19 < PDI < 0.30) and exhibiting a negative surface charge (−32 to −52 mV) were obtained as characterized by DLS, zeta potential, and TEM analyses. Using HepaRG and SK-MEL-28 cells, cytotoxicity studies evidenced no effect on cell viability at low concentrations (< 88 μg mL−1) while high concentrations (88–320 μg mL−1) induced a mild toxicity. Also, high concentrations of nanoparticles (ca. 180 μg mL−1) slightly reduced DNA replication while apoptosis measured with DEVD-AMC caspase activity remained unaffected, thus suggesting a moderate cytostatic effect of the nanoparticles without induction of cell death. Furthermore, HepaRG cells were found to efficiently uptake PMLA-b-PHB-based nanoparticles. All these results demonstrated that PMLA-b-PHB copolymers are promising candidates as drug-delivery systems.

 Please wait while we load your content...
Please wait while we load your content...