Preparation of multiformable supramolecular gels through helical complexation by amylose in vine-twining polymerization†
Abstract
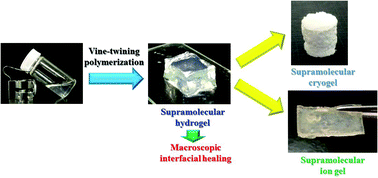
In this study, we prepared mutiformable functional supramolecular gels by vine-twining polymerization using poly(γ-glutamic acid-graft-ε-caprolactone) (PGA-g-PCL) as a new guest polymer, with subsequent procedures of lyophilization and exchange of dispersion media. When the phosphorylase-catalyzed enzymatic polymerization of the monomer α-D-glucose 1-phosphate from a maltoheptaose primer was carried out in the presence of PGA-g-PCL according to the vine-twining polymerization method, a supramolecular hydrogel was obtained. The resulting hydrogel, purified by soaking in water, had the self-standing properties. Macroscopic interfacial healing was achieved by the formation of inclusion complexes at the interface between two hydrogel pieces through enzymatic polymerization. Cryogels were obtained by the lyophilization of the hydrogels; XRD analysis of the cryogel indicated the presence of inclusion complexes of amylose with PCL graft chains in intermolecular (PGA-g-PCL)s, which acted as cross-linking points for hydrogelation. Porous morphologies were seen in scanning electron micrographs of the cryogels. Furthermore, ion gels were fabricated by soaking the hydrogels in the ionic liquid of 1-butyl-3-methylimidazolium chloride. The mechanical properties of the cryo- and ion gels were evaluated by compressive and tensile testing, respectively.

 Please wait while we load your content...
Please wait while we load your content...