Photoinduced topological transformation of cyclized polylactides for switching the properties of homocrystals and stereocomplexes†
Abstract
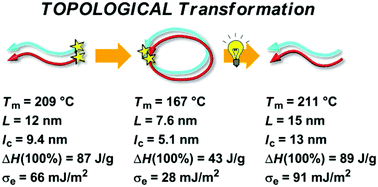
Cyclized poly(L-lactide) and poly(D-lactide) (Mn ∼ 3 kDa) incorporating an o-nitrobenzyl group as a photocleavable linker were synthesized and photoirradiated for topological transformation to form photocleaved linear polylactides. By DSC, Tm of the cyclized stereocomplex (167 °C) decreased by more than 40 °C from that of the linear prepolymers (209 °C) despite their essentially identical molecular weights. Upon the photocleavage, the resulting linear stereocomplex showed almost the same Tm (211 °C) as that before the cyclization. The enthalpy of melting of crystals having an infinite thickness, i.e. ΔHm(100%), and the surface free energy (σe) were determined by the combination of WAXD, SAXS, and DSC. Both ΔHm(100%) and σe were considerably smaller for the cyclized polylactide homocrystals and stereocomplexes than those of the linear prepolymers and photocleaved products. These suggest that the absolute enthalpy of the melt state is lower, and the crystalline–amorphous interface is more stable for the cyclized polylactides arising from the topology.

 Please wait while we load your content...
Please wait while we load your content...