Extent of intramolecular cyclization in RAFT-synthesized methacrylic branched copolymers using 13C NMR spectroscopy†
Abstract
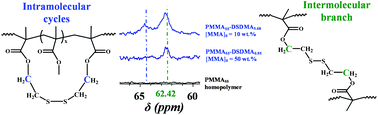
Recently, we reported using 1H NMR spectroscopy to assess the degree of intramolecular cyclization in a series of soluble methacrylic branched copolymers (see J. Rosselgong and S. P. Armes, Macromolecules, 2012, 45, 2731–2737). The key to success in addressing this long-standing problem in polymer science was the selection of a suitable disulfide-based dimethacrylate as the branching comonomer, which was statistically copolymerized with methyl methacrylate using reversible addition-fragmentation chain transfer (RAFT) polymerization. In this earlier work, estimation of the degree of intramolecular cyclization required peak deconvolution of the relevant thiamethylene proton signals. In the present work, we show that quantitative 13C NMR spectroscopy can also be utilized to determine the intramolecular cyclization for the same series of methacrylic copolymers via peak deconvolution of the oxymethylene carbon signals. Although this technique requires long spectral accumulation times, it offers superior resolution compared to 1H NMR spectroscopy and hence may ultimately enable this analytical approach to be applied to less esoteric divinyl comonomers.

 Please wait while we load your content...
Please wait while we load your content...