Efficient synthesis of diverse well-defined functional polypropylenes with high molecular weights and high functional group contents via thiol–halogen click chemistry†
Abstract
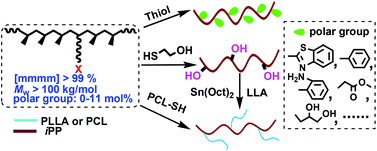
The thiol–halogen click chemistry between halogenated isotactic polypropylenes (iPPs) and thiols was systematically investigated for the first time. The order of copolymer reactivity can be summarized as iodinated ≫ brominated > chlorinated iPP, while for different thiols, the reactivity depended on not only the acidity of the sulfhydryl but also the competition of the polar group in the thiols with –SH to participate in the nucleophilic substitution reaction. In this case, various polar groups including hydroxyl, ester, aryl, thiazolyl and amino have been successfully introduced into iPP in a quantitative way, by employing iodinated iPP as the highly reactive intermediate. The content of polar groups in the high molecular weight functional iPPs (Mw > 100 kg mol−1) could be tuned in a wide range of 0–11 mol%. More notably, this new strategy offers an effective route to prepare the well-defined graft copolymer iPP-g-PCL from the halogen functionalized iPP and poly(ε-caprolactone)s with highly reactive –SH end (PCL-PhSH). Initiated by the resulting hydroxyl-containing iPP, ring-opening polymerization of L-lactide (LLA) provided another kind of graft copolymer, iPP-g-PLLA.

 Please wait while we load your content...
Please wait while we load your content...