Synthesis and post-polymerization reaction of end-clickable stereoregular polymethacrylates via termination of stereospecific living anionic polymerization
Abstract
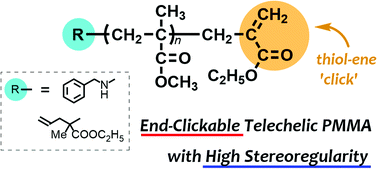
Poly(methyl methacrylate)s with high stereoregularity and clickable end-groups were synthesized via terminating reactions with α-(halomethyl)acrylates in stereospecific living anionic polymerization. The terminating reaction was efficient and tolerant to the reaction conditions to such an extent that almost quantitative end-functionalization was achieved in isotactic- and syndiotactic-specific polymerization systems. The terminating reactions were also achieved in polymerizations of vinyl methacrylate and trimethylsilyl methacrylate. For the polymerization of butyl acrylate, however, the termination efficiency was limited to less than 69%. Furthermore, the quantitative end-functionalization of the incorporated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond at ω-end was achieved with various thiols catalyzed by Et3N. The base-catalysed thiol–ene reaction of the stereoregular poly(vinyl methacrylate) with a ω-end C
C double bond at ω-end was achieved with various thiols catalyzed by Et3N. The base-catalysed thiol–ene reaction of the stereoregular poly(vinyl methacrylate) with a ω-end C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond selectively proceeded to retain vinyl ester functions, and the subsequent hydrolysis afforded ω-functional stereoregular poly(methacrylic acid). A combination of the terminating agent with a protected lithium amide afforded stereoregular poly(methyl methacrylates) with orthogonally clickable α- and ω-ends.
C double bond selectively proceeded to retain vinyl ester functions, and the subsequent hydrolysis afforded ω-functional stereoregular poly(methacrylic acid). A combination of the terminating agent with a protected lithium amide afforded stereoregular poly(methyl methacrylates) with orthogonally clickable α- and ω-ends.

 Please wait while we load your content...
Please wait while we load your content...