Novel thermo-sensitive hydrogels containing polythioether dendrons: facile tuning of LCSTs, strong absorption of Ag ions, and embedment of smaller Ag nanocrystals
Abstract
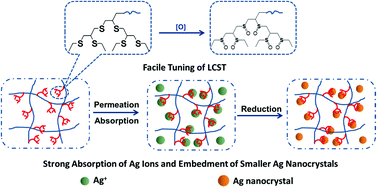
A novel “clawlike” dendritic monomer, 3, 2,3-bis((2,3-bis(ethylthio)propyl)-thio)propyl methacrylate was prepared and successfully incorporated into a thermosensitive poly(N-isopropylacrylamide) (PNIPAM) hydrogel system by free radical polymerization. The lower critical solution temperature (LCST) of these hydrogels can be altered in the range of 31.0 °C to 81.1 °C by varying the amount of dendritic monomers and oxidants. Fourier transform infrared spectroscopy (FTIR) demonstrated the successful preparation of hydrogels and the oxidation of the hydrogels. X-ray photoelectron spectroscopy (XPS) was used to confirm the molar ratio of thioether and sulfoxide groups. The hydrogels containing thioether or sulfoxide groups show excellent absorption towards Ag+ ions, and every dendritic monomer and its oxidized derivative can capture on average 1.31 and 1.62 Ag+ ions, respectively. These Ag+ ions in the hydrogel were in situ reduced by NaBH4. Thioether or sulfoxide groups in the hydrogel made the size of Ag nanoparticles (about 20 nm) much smaller than those nanoparticles in PNIPAM hydrogel. These hydrogels containing thioether groups as oxidant scavengers and broad spectrum antimicrobial Ag nanoparticles may have promising application in dealing with infected wounds.

 Please wait while we load your content...
Please wait while we load your content...