Multicomponent reactions of methyl substituted all-cis tetrafluorocyclohexane aldehydes†
Abstract
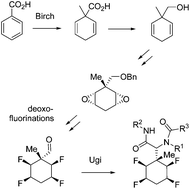
This paper reports the preparation of methyl substituted all-cis tetrafluorocyclohexanes prepared from a Birch reduction of benzoic acid, worked up with a methyl iodide quench. The resultant methylcyclohexadiene carboxylic acid was reduced to the alcohol, protected as an ether and then a sequence of functional group manipulations carried out to introduce four fluorines. The cyclohexadienyl ring was then epoxidised and the C–O bonds sequentially converted through deoxyfluorination reactions to two sets of isomers of all-cis tetrafluorocyclohexane isomers. The blocking methyl group renders the ring safe to hydrogen fluoride elimination. Deprotection of the benzylic ether and then oxidation gave aldehydes which were then used in Ugi and Passerini multicomponent reactions, allowing this facially polarised cyclohexane to be incorporated into peptidic structural motifs.

 Please wait while we load your content...
Please wait while we load your content...