Stereoselective recognition of the Ac-Glu-Tyr-OH dipeptide by pseudopeptidic cages†
Abstract
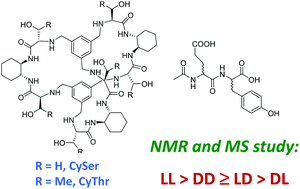
Pseudopeptidic molecular cages are appealing receptors since they can display different polar and non-polar interaction sites in a modular framework and a controlled disposition. Inspired by previous host–guest knowledge, two pseudopeptidic molecular cages based on serine and threonine (CySer and CyThr, respectively) were designed and synthesized as hosts for the binding of the four possible stereoisomers of the Ac-Glu-Tyr-OH dipeptide, a target sequence of tyrosine kinases. The careful NMR titration experiments in aqueous acetonitrile allowed the determination of the binding constants and reflected a difference in the stability of the corresponding diastereomeric host–guest complexes. The CySer cage proved to be slightly more efficient than the CyThr counterpart, although both showed similar stereoselectivity trends: LL > DD ≥ LD > DL. This stereoselective binding was retained in the gas phase, as shown by ESI-MS competition experiments using the enantiomer-labelled method (EL), as well as CID experiments. Thus, the MS-determined discriminations follow the same trends observed by NMR, suggesting that the stereoselectivity observed for these systems must be mainly dictated by the polar host–guest interactions. Despite the stereoselective binding of short peptide sequences in competitive media being a challenging issue in supramolecular chemistry, our results demonstrate the power of pseudopeptidic cages in molecular recognition with foreseen implications in chemical biology.

 Please wait while we load your content...
Please wait while we load your content...