Efficient Rh-catalyzed C–H borylation of arene derivatives under photochemical conditions†
Abstract
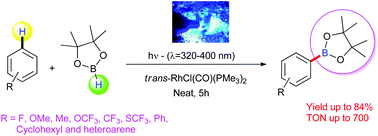
Photocatalysis allows innovations in organic synthesis. Among the various catalytic reactions, CH-functionalizations offer valuable possibilities for the refinement of easily available building blocks. In this respect, catalytic borylation is of interest, too. So far, most of the catalytic borylation reactions are performed under thermal conditions at comparably high temperatures. Here, we describe a new synthetic route for efficient borylation reactions of arenes using a photocatalytic pathway. This novel approach allows the synthesis of a broad variety of borylated arenes and heteroarenes under mild conditions. Applying trans-[Rh(PMe3)2(CO)Cl] as an active photocatalyst and HBPin as an boron source, we achieved high TON. A catalytic cycle that relies on a Rh(I)–Rh(III) interconversion is proposed.

 Please wait while we load your content...
Please wait while we load your content...