Bioorthogonal phase-directed copper-catalyzed azide–alkyne cycloaddition (PDCuAAC) coupling of selectively cross-linked superoxide dismutase dimers produces a fully active bis-dimer†
Abstract
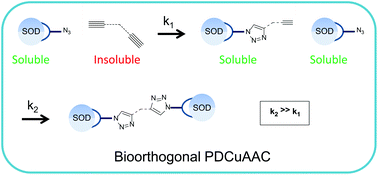
Superoxide dismutase (SOD) is a 32 kDa dimeric enzyme that actively removes a toxic oxygen species within red cells. The acellular protein itself does not survive circulation as it is filtered through the kidney. Conjugating the protein to another SOD should increase the size of the dual protein above the threshold for filtration by the kidney, making the material a potential therapeutic in circulation. Site-selective chemical cross-linking of SOD introduces a bioorthogonal azide group on the cross-link so that two SODs react efficiently with a bis-alkyne through phase-directed copper-catalyzed azide–alkyne cycloaddition (PDCuAAC). The modification has a negligible effect on the catalytic activity of the constituent proteins. Consistent with the retained activity, circular dichroism (CD) spectroscopy indicates that the secondary structures of the proteins are similar to that of the native protein.

 Please wait while we load your content...
Please wait while we load your content...