Highly chemoselective synthesis of dimeric 2-oxindoles with a C-3/C-5′ linkage via Friedel–Crafts alkylations of 2-oxindoles with 3-hydroxy-2-oxindoles†‡
Abstract
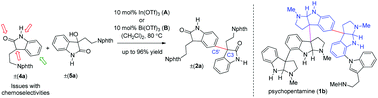
Simple and convenient Lewis acid-catalyzed Friedel–Crafts alkylations of 2-oxindoles as electron rich aromatics with 3-hydroxy-2-oxindoles as electron deficient partners have been developed. The reaction afforded a variety of dimeric 2-oxindoles with a C-3/C-5′ linkage having an all-carbon quaternary center at the pseudobenzylic position in high yields.

 Please wait while we load your content...
Please wait while we load your content...