Benzyl anion transfer in the fragmentation of N-(phenylsulfonyl)-benzeneacetamides: a gas-phase intramolecular SNAr reaction†
Abstract
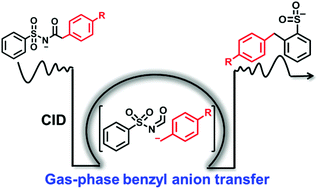
In this study, we report a gas-phase benzyl anion transfer via intramolecular aromatic nucleophilic substitution (SNAr) during the course of tandem mass spectrometry of deprotonated N-(phenylsulfonyl)-benzeneacetamide. Upon collisional activation, the formation of the initial ion/neutral complex ([C6H5CH2−/C6H5SO2NCO]), which was generated by heterolytic cleavage of the CH2–CO bond, is proposed as the key step. Subsequently, the anionic counterpart, benzyl anion, is transferred to conduct the intra-complex SNAr reaction. After losing neutral HNCO, the intermediate gives rise to product ion B at m/z 231, whose structure is confirmed by comparing the multistage spectra with those of deprotonated 2-benzylbenzenesulfinic acid and (benzylsulfonyl)benzene. In addition, intra-complex proton transfer is also observed within the complex [C6H5CH2−/C6H5SO2NCO] to generate product ion C at m/z 182. The INC-mediated mechanism was corroborated by theoretical calculations, isotope experiments, breakdown curve, substituent experiments, etc. This work may provide further understanding of the physicochemical properties of the gaseous benzyl anion.

 Please wait while we load your content...
Please wait while we load your content...