Synthesis and characterisation of a mesocyclic tripodal triamine ligand†
Abstract
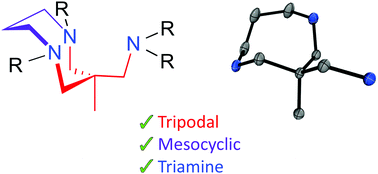
Meso- and macrocyclic polydentate amine ligands have been widely explored in oxidation catalysis and for the stabilization of unstable metal-superoxide, -peroxide, and -oxo intermediates. Herein we report on the design and synthesis of a novel mesocyclic, tripodal, triamine ligand that we believe will be an excellent addition to this field. We explored a number of synthetic procedures towards the mesocyclic asymmetric tetraalkylated ligand 1. We expect that 1 will bind metals in a facially capping manner, yielding complexes that display pseudo-tetrahedral geometry, potentially providing access to unprecedented late transition metal-oxo complexes (metal = Co, Ni, Cu). We describe the preparation of a library of mesocyclic polyamine synthons (8, 16, 17, 18, 19) that are precursors in the synthesis of 1. These synthons will be used to tailor the electronic properties of metal complexes of 1 and derivatives thereof. The X-ray crystal structures of 19 and mono- and di-protonated forms of 1b show that the triamine crystalises in a boat–chair conformation which is undesirable for metal coordination. However, solution 1H NMR studies show that in solution both 19 and the tetraalkylated derivative 1b are remarkably flexible. 1b reacted with [CuI(NCCH3)4](OTf) yielding a 1 : 1 copper(I) complex [CuI(NCCH3)(1b)]+.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners

 Please wait while we load your content...
Please wait while we load your content...