One probe, two-channel imaging of nuclear and cytosolic compartments with orange and red emissive dyes†
Abstract
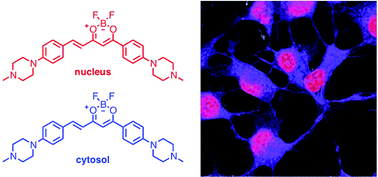
Several new DNA-targeting probes that exhibit binding-induced ‘turn on’ fluorescence are presented. Two of the dyes, orange emissive 1, (E)-4-(4(-4-methylpiperazin-1-yl)phenyl)6-(4-(4-methylpi-perazin-1-yl)styryl)pyrimidin-2-ol), and red emissive 2, (E)-4-(4(-4-methyl-piperazin-1-yl)-phenyl)6-(4-(4-methylpiperazin-1-yl)styryl)-1,3-propanedionato-κO,κO′]difluoroborane), are brightly fluorescent when bound to DNA, but are virtually non-fluorescent in aqueous solutions. Confocal fluorescence microscopy of live BT474, MCF7 and HEK293 cells demonstrates that both probes are cell permeable and rapidly accumulated intracellularly into cell nuclei and the cytosol. Taking advantage of their environmental sensitivity, these two pools of fluorophores are readily resolved into separate channels, and thus, a single dye allows two-color imaging of the nuclear and cytosolic compartments.

 Please wait while we load your content...
Please wait while we load your content...