Strategy of total synthesis based on the use of Rh-catalyzed stereoselective 1,4-addition
Abstract
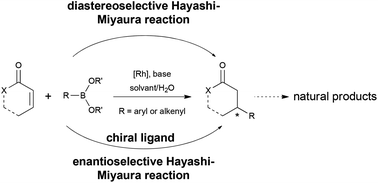
In 1998, Hayashi and Miyaura reported the first asymmetric conjugate addition of aryl- and alkenyl-boronic acids to α,β-unsaturated ketones using chiral rhodium complexes as catalysts. During the last decade, this reaction has been developed quickly and the enantioselectivity was significantly improved with the emergence of new phosphine ligands. In addition to the methodological work, this reaction was applied as a key step in the total synthesis of natural compounds. The purpose of this paper focuses on examples of the use of this reaction to prepare elaborated chiral molecules with high diastereoselectivies and/or enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...