Cinchona alkaloid-based chiral catalysts act as highly efficient multifunctional organocatalysts for the asymmetric conjugate addition of malonates to nitroolefins†
Abstract
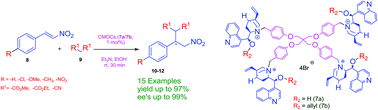
New pentaerythritol tetrabromide-based chiral quaternary ammonium salts acting as organocatalysts (7a and 7b) have been prepared and used as organocatalysts for enantioselective Michael addition reactions between various nitroolefins and Michael donors (malonates) under mild reaction conditions, such as lower concentration of base and catalyst and room temperature, with very good chemical yields (up to 97%) and ee's (up to 99%).

 Please wait while we load your content...
Please wait while we load your content...