Total synthesis of the natural product EBC-329†
Abstract
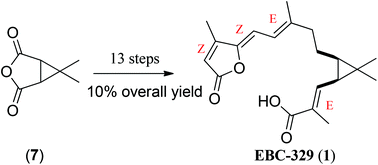
The first total synthesis of an anti-leukemic diterpene natural product EBC-329 (1) has been accomplished starting from readily available 6,6-dimethyl-3-oxabicyclo[3.1.0]hexane-2,4-dione (7). An efficient and general approach has been reported for the synthesis of EBC-329 in 13 steps with an overall yield of 10%.

 Please wait while we load your content...
Please wait while we load your content...