Synthesis of a new class of iminosugars based on constrained azaspirocyclic scaffolds by way of catalytic C–H amination†
Abstract
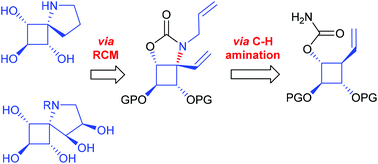
The synthesis of the first examples of a new class of iminosugars based on constrained spirocyclic scaffolds has been achieved via Rh-catalyzed C(sp3)–H amination. In this process, the needed electronic control in securing high regioselectivity from substrates with a high density of activated C–H bonds was achieved by using a combination of activating and electron-withdrawing groups.

 Please wait while we load your content...
Please wait while we load your content...