A platform for efficient, thiol-stable conjugation to albumin's native single accessible cysteine†
Abstract
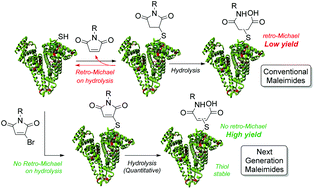
Herein we report the use of bromomaleimides for the construction of stable albumin conjugates via conjugation to its native, single accessible, cysteine followed by hydrolysis. Advantages over the classical maleimide approach are highlighted in terms of quantitative hydrolysis and absence of undesirable retro-Michael deconjugation.

 Please wait while we load your content...
Please wait while we load your content...