Colorful surface architectures with three different types of dynamic covalent bonds: integration of anthocyanins, tritylium ions and flavins†
Abstract
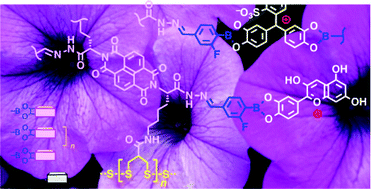
Although they combine the best of covalent and non-covalent bonds, dynamic covalent bonds are usually not used together. Building on pioneering examples for functional systems with two orthogonal dynamic covalent bonds, we herein elaborate on multicomponent surface architectures that operate with three different types of dynamic covalent bonds. Disulfide exchange under basic conditions is used to grow single π stacks directly on oxide surfaces, hydrazone exchange under acidic conditions to add a second string or stack, and boronic-ester exchange under neutral conditions to build the third one. In this study, we show that this synthetic approach to complex systems provides access to emergent properties, as exemplified with ordered stacks of anthocyanins, pyrocatchol violet and riboflavins. The integration of anthocyanins, the central component of the pigments of plant flowers, is interesting to protect the blue flavylium cation against deprotonation, deplanarization and degradation. The integration of pyrocatchol violet is of interest to stabilize the blue, disfavored tritylium cation. The red riboflavin stacks are attractive because they generate high photocurrent. These colorful examples hint at the potential of synthetic methods that use three different types of dynamic covalent bonds in concert to build complex systems with emergent properties.

 Please wait while we load your content...
Please wait while we load your content...