Substrate and stereocontrolled iodocycloetherification of highly functionalized enantiomerically pure allylic alcohols: application to synthesis of cytotoxic 2-epi jaspine B and its biological evaluation†
Abstract
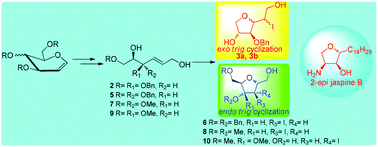
Stereoselectivities of electrophilic additions of molecular iodine to enantiomerically pure highly functionalized allylic alcohols with internal nucleophiles have been investigated. The intramolecular nucleophilic attack on the I2–π complex by an oxygen nucleophile to obtain tri- and tetrasubstituted THFs is highly regio-, stereoselective and substrate controlled. The application of this study has been shown by utilizing one of the THFs 4a as a key intermediate to complete the total synthesis of marine anti-cancer natural product 2-epi jaspine B.

 Please wait while we load your content...
Please wait while we load your content...