New PKS-NRPS tetramic acids and pyridinone from an Australian marine-derived fungus, Chaunopycnis sp.†
Abstract
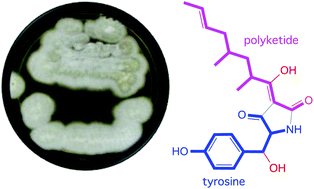
Chemical analysis of a marine-derived fungus, Chaunopycnis sp. (CMB-MF028), isolated from the inner tissue of a pulmonate false limpet Siphonaria sp., collected from rock surfaces in the intertidal zone of Moora Park, Shorncliffe, Queensland, yielded the tetramic acid F-14329 (1) and new analogues, chaunolidines A–C (2–4), together with the new pyridinone chaunolidone A (5), and pyridoxatin (6). Structures inclusive of absolute configurations were assigned to 1–6 on the basis of detailed spectroscopic analysis, X-ray crystallography, electronic circular dichroism (ECD), biosynthetic considerations and chemical interconversion. Chaunolidine C (4) exhibits modest Gram-positive antibacterial activity (IC50 5–10 μM), while chaunolidone A (5) is a selective and potent inhibitor (IC50 0.09 μM) of human non-small cell lung carcinoma cells (NCI-H460). Tetramic acids 1–4 form metal chelates with Fe(III), Al(III), Cu(II), Mg(II) and Zn(II).

 Please wait while we load your content...
Please wait while we load your content...