A theoretical investigation of substituent effects on the stability and reactivity of N-heterocyclic olefin carboxylates†
Abstract
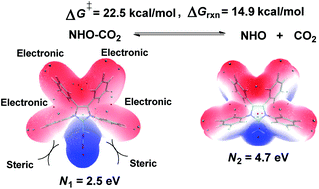
A theoretical study of substituent effects on the stability and reactivity of novel synthesized N-heterocyclic olefin (NHO) carboxylates has been performed using a combination of density functional theory (DFT) calculations, molecular electrostatic potential (MESP) minimum and nucleophilicity index analyses. These calculations demonstrate that the nucleophilicity of free NHO is stronger than that of the NHO–CO2 adduct and, hence, the thermally unstable NHO–CO2 adduct should be a more efficient organocatalyst for nucleophile-mediated reactions. The stability of the NHO–CO2 adduct, as well as the reactivity of free NHO, is strongly dependent on the electronic and steric effects of the C- and N-substituents on the imidazole ring. This dependency is reflected by the measured MESP minimum for the carboxylate moiety, the NHO–CO2 adduct (Vmin1), and the terminal carbon atom of free NHO (Vmin2). C-Substituents exert only electronic effects while N-substituents exert both electronic and steric effects. In general, the electron-withdrawing groups on the C- and N-positions favor decarboxylation while weakening the reactivity of NHO. These positions favor decarboxylation due to the simultaneous decrease of the electronic density on the carboxyl moiety of the NHO–CO2 and the terminal carbon atom of olefins. Additionally, the balance between the stability of the NHO–CO2 and the reactivity of free NHO can be tuned by the combined effects of the C- and N-substituents. The introduction of weak electron-withdrawing groups at the C-position and aromatic substituents or similar ring-strained entities at the N-position favors decarboxylation of the NHO–CO2 adduct and ensures the free NHO as a strong nucleophile.

 Please wait while we load your content...
Please wait while we load your content...