Synthesis of 8-hydroxy-2-iminochromene derivatives as selective and potent inhibitors of human carbonyl reductase 1†
Abstract
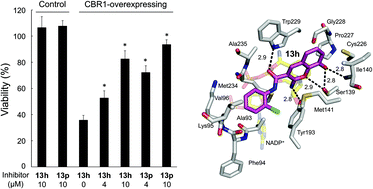
Human carbonyl reductase 1 (CBR1), a member of the short-chain dehydrogenase/reductase superfamily, reduces anthracycline anticancer drugs to their less potent anticancer C-13 hydroxy metabolites, which are linked with pathogenesis of cardiotoxicity, a side effect of the drugs. CBR1 inhibitors are thought to be promising agents for adjuvant therapy with a twofold beneficial effect in prolonging the anticancer efficacy of the anthracyclines while decreasing cardiotoxicity. In order to search for new potential inhibitors of CBR1, we synthesized a series of des-methoxyphenyl derivatives of (Z)-2-(4-methoxyphenylimino)-7-hydroxy-N-(pyridin-2-yl)-2H-chromene-3-carboxamide (1) that was developed previously as a potent inhibitor of aldo-keto reductase (AKR) 1B10 and AKR1B1. Among the newly synthesized inhibitors, 8-hydroxy-2-imino-2H-chromene-3-carboxylic acid (2-chlorophenyl)amide (13h) was the most potent competitive inhibitor of CBR1, showing a Ki value of 15 nM. 13h also showed high selectivity to CBR1 over its isozyme CBR3 and other enzymes with CBR activity (AKR1B1, AKR1B10, AKR1C1, AKR1C2, AKR1C4, DXCR and DHRS4). Furthermore, 13h inhibited the cellular metabolism by CBR1 at its concentration of 4 μM. The structure–activity relationship of the derivatives, site-directed mutagenesis of putative binding residues (Met141 and Trp229) and molecular docking of 13h in CBR1 revealed that the interactions of 13h with the substrate-binding residues (Ser139, Met141, Tyr193 and Trp229) are important for the tight binding.


 Please wait while we load your content...
Please wait while we load your content...