Direct access to site-specifically phosphorylated-lysine peptides from a solid-support†
Abstract
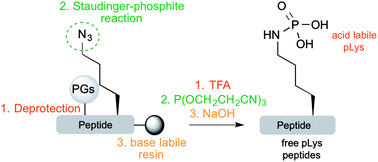
Phosphorylation is a key process for changing the activity and function of proteins. The impact of phospho-serine (pSer), -threonine (pThr) and -tyrosine (pTyr) is certainly understood for some proteins. Recently, peptides and proteins containing N-phosphorylated amino acids such as phosphoarginine (pArg), phosphohistidine (pHis) and phospholysine (pLys) have gained interest because of their different chemical properties and stability profiles. Due to its high intrinsic lability, pLys is the least studied within this latter group. In order to gain insight into the biological role of pLys, chemical and analytical tools, which are compatible with the labile P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–N bond, are highly sought-after. We recently reported an in-solution synthetic approach to incorporate pLys residues in a site-specific manner into peptides by taking advantage of the chemoselectivity of the Staudinger-phosphite reaction. While the in-solution approach allows us to circumvent the critical TFA cleavage, it still requires several transformations and purification steps to finally deliver pLys peptides. Here we report the synthesis of site-specific pLys peptides directly from a solid support by using a base labile resin. This straightforward and highly efficient approach facilitates the synthesis of various site-specific pLys-containing peptides and lays the groundwork for future studies about this elusive protein modification.
O)–N bond, are highly sought-after. We recently reported an in-solution synthetic approach to incorporate pLys residues in a site-specific manner into peptides by taking advantage of the chemoselectivity of the Staudinger-phosphite reaction. While the in-solution approach allows us to circumvent the critical TFA cleavage, it still requires several transformations and purification steps to finally deliver pLys peptides. Here we report the synthesis of site-specific pLys peptides directly from a solid support by using a base labile resin. This straightforward and highly efficient approach facilitates the synthesis of various site-specific pLys-containing peptides and lays the groundwork for future studies about this elusive protein modification.

 Please wait while we load your content...
Please wait while we load your content...