Facile synthesis of 4- and 7-azaindoles from the corresponding imines by palladium-catalyzed cascade C–C and C–N coupling†
Abstract
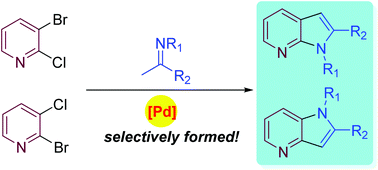
The cyclization of 2,3-dihalopyridines with readily available imines provides a convenient and regioselective approach to 4- and 7-azaindoles. The regioselectivity can be controlled by the choice of the halogen atoms at the pyridine ring (chlorine versus bromine).

 Please wait while we load your content...
Please wait while we load your content...