Corrole and nucleophilic aromatic substitution are not incompatible: a novel route to 2,3-difunctionalized copper corrolates†
Abstract
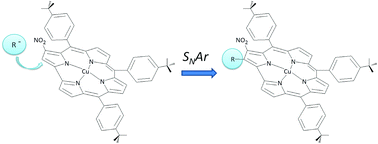
The insertion of a –NO2 group onto the corrole framework represents a key step for subsequent synthetic manipulation of the macrocycle based on the chemical versatility of such a functionality. Here we report results of the investigation of a copper 3-NO2-triarylcorrolate in nucleophilic aromatic substitution reactions with “active” methylene carbanions, namely diethyl malonate and diethyl 2-chloromalonate. Although similar reactions on nitroporphyrins afford chlorin derivatives, nucleophilic attack on carbon-2 of corrole produces 2,3-difunctionalized Cu corrolates in acceptable yields (ca. 30%), evidencing once again the erratic chemistry of this contracted porphyrinoid.

 Please wait while we load your content...
Please wait while we load your content...