Gold-catalyzed cascade C–H/C–H cross-coupling/cyclization/alkynylation: an efficient access to 3-alkynylpyrroles†
Abstract
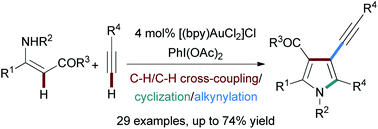
An efficient approach to 3-alkynylpyrroles has been developed through the gold-catalyzed reaction of β-enamino derivatives with terminal alkynes, which features complete regiocontrol, relatively wide substrate scope, and high functional group tolerance. Mechanistic studies show that the reaction proceeds through the gold-catalyzed cascade oxidative C–H/C–H cross-coupling, cyclization and alkynylation.

 Please wait while we load your content...
Please wait while we load your content...