Studies on the synthesis, stability and conformation of 2-sulfonyl-oxetane fragments†
Abstract
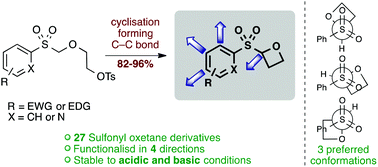
2-(Arylsulfonyl)oxetanes have been prepared as new structural motifs of interest for medicinal chemistry. These are designed to fit within fragment space and be suitable for screening in fragment based drug discovery, as well as being suitable for further elaboration or incorporation into drug-like compounds. The oxetane ring is constructed through an efficient C–C bond forming cyclisation which allows the incorporation of a wide range of aryl-sulfonyl groups. Furthermore, biaryl-containing compounds can be accessed through Suzuki–Miyaura coupling from halogenated derivatives. With a number of oxetane containing fragment compounds available, their pH stability was assessed, indicating good half-life values for mono-substituted aryl sulfonyl oxetanes across the pH range (1 to 10). Solubility and metabolic stability data is also reported. Finally, the conformation of the fragments is assessed computationally, providing an indication of possible binding orientations.

 Please wait while we load your content...
Please wait while we load your content...