Synthesis of naked amino-pyrroloindoline via direct aminocyclization of tryptamine†
Abstract
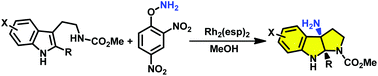
The first direct access to unprotected amino-pyrroloindoline via aminocyclization of tryptamine and tryptophan has been described. A variety of structurally diverse amino-pyrroloindolines are furnished by the use of O-(2,4-dinitrophenyl)hydroxylamine (DPH) as the nitrogen source in the presence of catalytic Rh2(esp)2.

 Please wait while we load your content...
Please wait while we load your content...