Synergism between genome sequencing, tandem mass spectrometry and bio-inspired synthesis reveals insights into nocardioazine B biogenesis†
Abstract
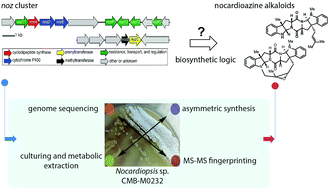
Marine actinomycete-derived natural products continue to inspire chemical and biological investigations. Nocardioazines A and B (3 and 4), from Nocardiopsis sp. CMB-M0232, are structurally unique alkaloids featuring a 2,5-diketopiperazine (DKP) core functionalized with indole C3-prenyl as well as indole C3- and N-methyl groups. The logic of their assembly remains cryptic. Bioinformatics analyses of the Nocardiopsis sp. CMB-M0232 draft genome afforded the noz cluster, split across two regions of the genome, and encoding putative open reading frames with roles in nocardioazine biosynthesis, including cyclodipeptide synthase (CDPS), prenyltransferase, methyltransferase, and cytochrome P450 homologs. Heterologous expression of a twelve gene contig from the noz cluster in Streptomyces coelicolor resulted in accumulation of cyclo-L-Trp-L-Trp DKP (5). This experimentally connected the noz cluster to indole alkaloid natural product biosynthesis. Results from bioinformatics analyses of the noz pathway along with challenges in actinomycete genetics prompted us to use asymmetric synthesis and mass spectrometry to determine biosynthetic intermediates in the noz pathway. The structures of hypothesized biosynthetic intermediates 5 and 12–17 were firmly established through chemical synthesis. LC-MS and MS-MS comparison of these synthetic compounds with metabolites present in chemical extracts from Nocardiopsis sp. CMB-M0232 revealed which of these hypothesized intermediates were relevant in the nocardioazine biosynthetic pathway. This established the early and mid-stages of the biosynthetic pathway, demonstrating that Nocardiopsis performs indole C3-methylation prior to indole C3-normal prenylation and indole N1′-methylation in nocardioazine B assembly. These results highlight the utility of merging bioinformatics analyses, asymmetric synthetic approaches, and mass spectrometric metabolite profiling in probing natural product biosynthesis.


 Please wait while we load your content...
Please wait while we load your content...