Palladium-catalyzed carbonylative Sonogashira coupling between aryl triazenes and alkynes†
Abstract
We developed a palladium-catalyzed carbonylative Sonogashira reaction with aryl triazenes and alkynes as substrates and methanesulfonic acid as the additive. A series of α,β-ynones were synthesized by this alternative procedure. Notably, bromides, iodides and hydroxyl groups could be well-tolerated under these reaction conditions.
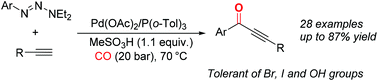

 Please wait while we load your content...
Please wait while we load your content...