C5-azobenzene-functionalized locked nucleic acid uridine: isomerization properties, hybridization ability, and enzymatic stability†
Abstract
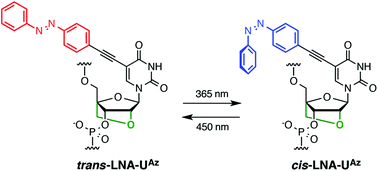
Oligonucleotides (ONs) modified with a locked nucleic acid (LNA) are widely used in the fields of therapeutics, diagnosis, and nanotechnology. There have been significant efforts towards developing LNA analogues bearing modified bridges to improve their hybridization ability, nuclease resistance, and pharmacokinetic profiles. Moreover, nucleobase modifications of LNA are useful strategies for the functionalization of ONs. Modifications of the C5-position of pyrimidine nucleobases are particularly interesting because they enable predictable positioning of functional groups in the major groove of the duplex. Here we report the synthesis of C5-azobenzene-functionalized LNA uridine (LNA-UAz) and properties of LNA-UAz-modified ONs, including isomerization properties, hybridization ability, and enzyme stability. LNA-UAz in ON is photo-isomerized effectively and reversibly by irradiation at 365 nm (trans to cis) and 450 nm (cis to trans). LNA-UAz-modified ONs show RNA-selective hybridization ability despite the large hydrophobic azobenzene moiety extending into the major groove of the duplex. The enzymatic stability of LNA-UAz-modified ONs is higher than that of natural and LNA-modified ONs with or without photo-irradiation. Our results indicate that LNA-UAz holds promise for RNA targeting and photo-switchable technologies.

 Please wait while we load your content...
Please wait while we load your content...