Functionalising the azobenzene motif delivers a light-responsive membrane-interactive compound with the potential for photodynamic therapy applications†
Abstract
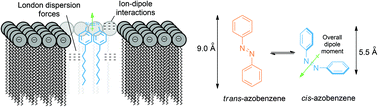
When adorned with n-octyl chains azobenzene is able to disrupt a variety of calcein-loaded phospholipid liposomes. The levels of lysis observed are dependent both on the lipid headgroup and the conformation of the azobenzene compound. In all cases studied, it has been shown that the cis-conformer is more membrane-interactive than the trans-conformer, suggesting that this class of molecule could be optimised for photo-dynamic therapy applications against infectious pathogens.

 Please wait while we load your content...
Please wait while we load your content...