Rhodium-catalyzed asymmetric addition of arylboronic acids to cyclic N-sulfonyl ketimines towards the synthesis of α,α-diaryl-α-amino acid derivatives†
Abstract
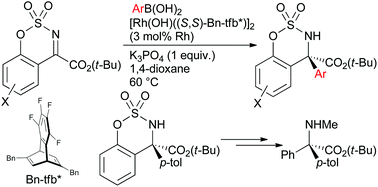
Rhodium/chiral diene complex-catalyzed asymmetric addition of arylboronic acids to cyclic ketimines having an ester group proceeded to give the corresponding α-amino acid derivatives in high yields with high enantioselectivity. The cyclic amino acid derivative was transformed into a linear α,α-diaryl-substituted α-N-methylamino acid ester.

 Please wait while we load your content...
Please wait while we load your content...