Divergent, stereoselective access to heterocyclic α,α-quaternary- and β2,3,3-amino acid derivatives from a N-Pmp-protected Orn-derived β-lactam†‡
Abstract
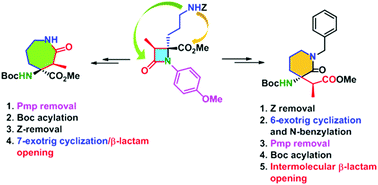
A suitably protected Orn-derived (3S,4S)-β-lactam was used as common intermediate in the synthesis of conformationally constrained (3S,4S)-2-oxoazepane α,α- and (2S,3S)-2-oxopiperidine-β2,3,3-amino acid derivatives. Compared to alternative procedures using an N-p-methoxybenzyl group at the 2-azetidinone, the incorporation of a p-methoxyphenyl moiety is crucial for the excellent stereochemical outcomes in the preparation of these heterocyclic amino acids. Chemoselective 7- or 6-exo-trig cyclization was achieved through alternative sequences of Pmp-deprotection/Boc-activation, followed by inter- and intramolecular β-lactam ring opening, respectively.

 Please wait while we load your content...
Please wait while we load your content...